Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation . Cu 2+ (aq) + 2ch3coo¯(aq) + ca. Complete ionic equations show dissolved ionic solids as separated. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. ionic compounds that dissolve separate into individual ions. in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,.
from spmscience.blog.onlinetuition.com.my
Cu 2+ (aq) + 2ch3coo¯(aq) + ca. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. enter an equation of an ionic chemical equation and press the balance button. what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. The balanced equation will be calculated along. Complete ionic equations show dissolved ionic solids as separated. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. ionic compounds that dissolve separate into individual ions. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,.
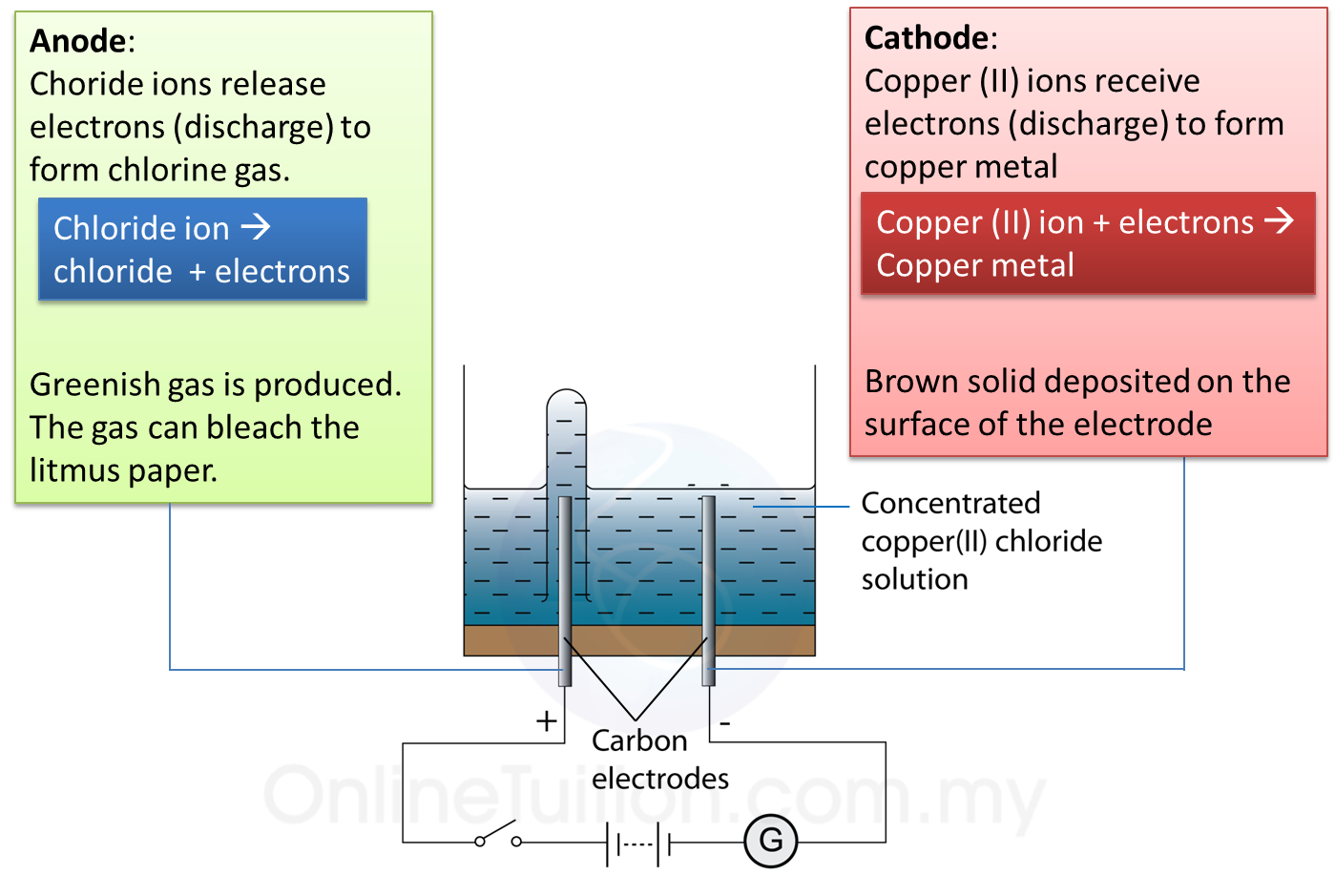
5.5.2 Electrolysis of Copper (II) Chloride Solution SPM Science
Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. ionic compounds that dissolve separate into individual ions. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. Complete ionic equations show dissolved ionic solids as separated. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. The balanced equation will be calculated along.
From www.youtube.com
How to Write the Net Ionic Equation for Cr 3+ and NaOH YouTube Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. The balanced equation will be calculated along. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. Complete ionic equations show. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. The balanced equation will be calculated along. ionic compounds that dissolve separate into individual. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. The balanced equation will be calculated along. in the following section, we will examine the reaction that occurs when a solid piece of. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. ionic compounds that dissolve separate into individual ions. Complete ionic equations show dissolved ionic solids as separated. learn for free about. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From spmscience.blog.onlinetuition.com.my
5.5.2 Electrolysis of Copper (II) Chloride Solution SPM Science Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation enter an equation of an ionic chemical equation and press the balance button. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. Complete ionic equations show dissolved ionic solids as separated. ionic compounds that dissolve separate into individual ions. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation The balanced equation will be calculated along. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. what are the total ionic and net ionic equations for copper(ii) acetate and. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? enter an equation of an ionic chemical equation and press the balance button. in the following section, we will examine the reaction that occurs when a solid. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From studylib.net
Practice Problems on Net Ionic Equations Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. enter an. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. Complete ionic equations show dissolved ionic solids as separated. ionic compounds that dissolve separate into individual ions. The balanced equation will be calculated along.. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From ar.inspiredpencil.com
Single Replacement Reaction Equation Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation Complete ionic equations show dissolved ionic solids as separated. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. The balanced equation will be calculated along. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. ionic. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation Complete ionic equations show dissolved ionic solids as separated. enter an equation of an ionic chemical equation and press the balance button. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. this net ionic equation indicates that solid silver chloride may be produced from dissolved. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.coursehero.com
[Solved] copper(II)sulfate + Barium chloride molecular equation and Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. The balanced equation will be calculated along. enter. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation what are the total ionic and net ionic equations for copper(ii) acetate and calcium chloride? learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. how to write the net ionic equation for cucl2. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From gbu-taganskij.ru
Net Ionic Equation And Complete Ionic Equation, 59 OFF Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. enter an equation of an ionic chemical equation and press the balance button. how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. in the following section, we will examine the reaction. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. in the following section, we will examine the reaction that occurs when a solid piece of elemental magnesium in placed in an. Complete ionic equations show dissolved ionic solids as separated. learn for free about math, art, computer. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. The balanced equation will be calculated along. ionic compounds that dissolve separate into individual ions. enter an equation of an ionic chemical equation and press the balance button. Complete ionic equations show dissolved ionic solids as separated. this net ionic equation indicates. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation how to write the net ionic equation for cucl2 + koh = cu (oh)2 + kcl. Cu 2+ (aq) + 2ch3coo¯(aq) + ca. ionic compounds that dissolve separate into individual ions. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance,. this net ionic equation indicates that solid silver chloride may be. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. enter an equation of an ionic chemical equation and press the balance button. this net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(i) ions,. learn for free about math, art,. Copper Ii Chloride And Potassium Hydroxide Net Ionic Equation.